
In the diagram above,  represents an atom of nitrogen and
represents an atom of nitrogen and 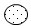 represents an atom of oxygen. What is the IUPAC name of this compound?
represents an atom of oxygen. What is the IUPAC name of this compound?
A. Nitrogen oxide
Incorrect. The IUPAC name for this compound should include prefixes to indicate the number of each type of atom.
B. Dinitrogen dioxide
Incorrect. While each compound in the diagram contains two nitrogen atoms, the prefix used to indicate the number of oxygen atoms is incorrect.
C. Tetranitrogen dioxide
Incorrect. The prefix used to indicate the number of nitrogen atoms in a simple compound is incorrect.
D. Dinitrogen tetroxide
Correct! Each compound contains two nitrogen atoms [di =2] and four oxygen atoms [tetr(a) = 4].
What is the name of the compound whose formula is Mg2N3?
A. Magnesium (II) nitride
Incorrect. Magnesium is a main group element, not a transition metal, and does not utilize the IUPAC nomenclature rules for transition metals.
B. Magnesium nitride
Correct! This follows the IUPAC nomenclature rules for binary ionic compounds.
C. Magnesium (III) nitride
Incorrect. Magnesium is not a transition metal and does not utilize the IUPAC nomenclature rules for transition metals.
D. Dimagnesium trinitride
Incorrect. The IUPAC nomenclature rules for binary ionic compounds do not utilize prefixes.
Which of the following pair of formulas and names do not match?
A. SiCl4 : tin (IV) chloride
Incorrect. The formula and name match.
B. MgSO3 : magnesium sulfate
Correct! SO3 represents the sulfite ion (SO32-). Therefore, the correct name would be magnesium sulfite.
C. CuO : copper (II) oxide
Incorrect. The formula and name match.
D. NH4OH : ammonium hydroxide
Incorrect. The formula and name match.
What is the name of the following chemical formula: PbF2?
A. Lead fluoride
Incorrect. This formula does not follow the IUPAC nomenclature rules for naming binary compounds that contain a transition metal.
B. Lead (II) fluoride
Correct! Lead is a multivalent, transition metal. As illustrated in the formula, it takes two fluoride ions (F-) to bond with one lead (II) ion (Pb2+). Multivalent ions use roman numerals.
C. Lead difluoride
Incorrect. The formula PbF2 follows IUPAC binary ionic nomenclature rules.
D. Lead fluoride (II)
Incorrect. The fluoride ion is a main group ion and does not utilize roman numerals.
Which of the following correctly represents the formula for the compound calcium hydroxide?
A. CaOH
Incorrect. The calcium ion has an oxidation number of +2 and the hydroxide ion has an oxidation number of -1.
B. CaOH2
Incorrect. This formula does not accurately represent the hydroxide ion as a bonded unit (OH-).
C. Ca(OH)2
Correct! This formula is a result of one calcium atom forming an ionic bond with two hydroxide ions.
D. Ca2(OH)
Incorrect. The hydroxide ion has an oxidation charge of -1.
If strontium and bromine were to form a neutral ionic compound, what ratio of each element is needed?
A. 1 strontium : 1 bromine
Incorrect. Strontium has an oxidation number of two.
B. 2 strontium : 1 bromine
Incorrect. The bromide ion has an oxidation number of -1. It would take two bromide ions to interact with one strontium ion.
C. 1 strontium : 2 bromine
Correct! Sr2+ and Br1- would results in the formula SrBr2 (one strontium ion for every two bromide ions) and a neutral ionic compound.
D. 3 strontium : 2 bromine
Incorrect. The ratios indicated would not result in a neutral ionic compound.
Which of the following pair of names and formulas do not match?
A. Sodium nitrate : NaNO3
Incorrect. The name and formula match. Na1+ and NO3 1- form NaNO3.
B. Lead (II) phosphate : Pb3(PO4)2
Incorrect. The name and formula match. Pb2+ and PO4 3- form Pb3(PO4)2.
C. Nitrous acid : HNO3
Correct! The name and formula do not match. Nitrous acid is the name given to the compound containing the nitrite ion (NO21-), not the nitrate ion (NO31-). The proper name for HNO3 would be nitric acid.
D. Potassium hydroxide : KOH
Incorrect. The name and formula match. K1+ and OH1- form KOH.
Which of the following is the IUPAC name for H3PO4?
A. Hydrophosphate
Incorrect. The name does not follow the conventions for IUPAC acid nomenclature based on the chemical formula.
B. Hydrogen triphosphate
Incorrect. The name does not follow the conventions for IUPAC acid nomenclature based on the chemical formula.
C. Phosphoric acid
Correct! The formula follows the conventions for IUPAC acid nomenclature.
D. Phosphorous acid
Incorrect. The polyatomic ion in this formula is phosphate (PO43-) not phosphite (PO33-).
The Lewis Dot Structure of a compound is shown below.
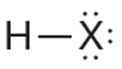
Which of the following elements does X represent in the structure?
A. Carbon
Incorrect. The dash represents a pair of electrons shared between both atoms. Prior to bonding, X contains seven valence electrons – six that are not bonded and one that is shared with hydrogen. Carbon only has four valence electrons.
B. Nitrogen
Incorrect. The dash represents a pair of electrons shared between both atoms. Prior to bonding, X contains seven valence electrons – six that are not bonded and one that is shared with hydrogen. Nitrogen only has five valence electrons.
C. Oxygen
Incorrect. The dash represents a pair of electrons shared between both atoms. Prior to bonding, X contains seven valence electrons – six that are not bonded and one that is shared with hydrogen. Oxygen only has six valence electrons.
D. Fluorine
Correct! Fluorine contains seven valence electrons and requires one more in its outer shell to be stable.
The Lewis Dot Structure of a compound is shown below.
Which of the following elements does X represent in the structure?
A. Lithium
Correct! The illustration shows that each X has an oxidation number of +1. Lithium is the only choice that forms an ion with a +1 charge. Additionally, the resulting formula is X2S. Lithium is the only choice that would best fit this.
B. Beryllium
Incorrect. The illustration shows that each X has an oxidation number of +1. Beryllium has an oxidation number of +2.
C. Fluorine
Incorrect. The illustration shows that each X has an oxidation number of +1. Fluorine has an oxidation number of -1.
D. Neon
Incorrect. The illustration shows that each X has an oxidation number of +1. Neon has an oxidation number of 0.
Which of the following is the correct Lewis Dot Structure for carbon tetrabromide?
A. CF4
Incorrect. This does not display a Lewis Dot Structure.
B.
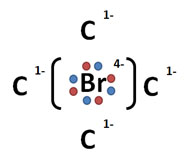
Incorrect. The Lewis Dot Structure for carbon tetrabromide should illustrate the sharing of electrons. Additionally, while this structure attempts to display an example of an ionic bond, it does so incorrectly as bromine does not have a charge of -4; carbon does not have a charge of +1.
C.
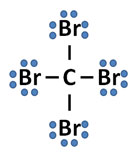
Correct! Each atom has eight valence electrons and the illustration properly shows how the atoms are covalently bonded.
D.
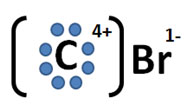
Incorrect. The Lewis Dot Structure for carbon tetrabromide should illustrate the sharing of electrons. Additionally, while this structure attempts to display an example of an ionic bond, it does so incorrectly.
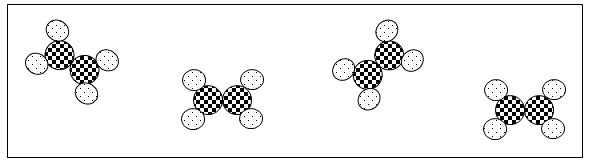
Which of the following models best represents the molecular shape of a compound with trigonal planar geometry?
A.
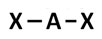
Incorrect. This answer choice reflects a linear molecular geometry.
B.
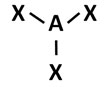
Correct! This answer choice reflects a trigonal planar molecular geometry.
C.
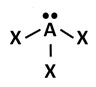
Incorrect. This answer choice reflects a trigonal pyramidal molecular geometry.
D.
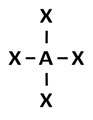
Incorrect. This answer choice reflects a tetrahedral molecular geometry.
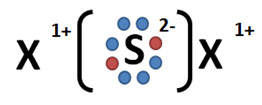
Which of the following models best represents the molecular shape of the ammonia molecule, NH3?
A.
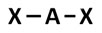
Incorrect. This answer choice only illustrates two bonding regions, whereas ammonia has three and has a lone pair.
B.
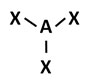
Incorrect. Ammonia has four electron regions. Initially this may seem correct, however, the model fails to account for ammonia’s lone pair of electrons, which cause the molecule to have a trigonal pyramidal molecular geometry.
C.
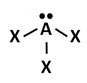
Correct! Ammonia has four electron regions – three bonding and one lone pair. As a result, ammonia’s molecular geometry is trigonal pyramidal.
D.
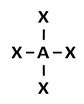
Incorrect. While ammonia has four electron regions, it does not have four bonding regions.
Which of the following best represents the molecular shape of the water molecule, H2O?
A. Bent
Correct! The water molecule has four areas of electron density – two bonding and two lone pairs. As a result, the molecular shape of the water molecule is bent.
B. Linear
Incorrect. The two lone pairs of electrons distort the molecule’s shape such that the molecule is not linear.
C. Trigonal planar
Incorrect. While the water molecule does have four areas of electron density, it does not have three bonding regions.
D. Tetrahedral
Incorrect. While the water molecule does have four areas of electron density, which would indicate a tetrahedral arrangement, this is the electron geometry shape not the molecular geometry.
Which of the following best explains the importance of the mole concept in chemistry?
A. The mole allows you to identify the atomic number of an element.
Incorrect. The atomic number is not determined by the mole concept.
B. The mole allows you to count atoms and molecules.
Correct! Atoms and molecules are too small to be seen with the visible eye; therefore, the mole concept allows you to count microscopic particles by weighing macroscopic amounts of substances.
C. The mole allows you to calculate the average atomic mass.
Incorrect. The average atomic mass is calculated by determining the percent abundance and mass of each isotope of an atom.
D. The mole allows you to determine the number of valence electrons.
Incorrect. The mole does not determine the number of valence electrons for an atom.
Which of the following amounts would yield the largest number of moles?
A. 10.0 grams of fluorine
Correct! 10.0 grams of fluorine would yield 0.52 moles.
B. 10.0 grams of sodium
Incorrect. 10.0 grams of sodium would yield 0.43 moles.
C. 10.0 grams of aluminum
Incorrect. 10.0 grams of aluminum would yield 0.37 moles.
D. 10.0 grams of strontium
Incorrect. 10.0 grams of strontium would yield 0.11 moles.
The students in Mrs. Smith’s chemistry class were instructed to obtain 5.00 moles of sodium chloride from a container as part of their lab practical. Which of the following best illustrates how the students would achieve this?
A.
B.
C.
D.
How many atoms of hydrogen are in two moles of methane, CH4?
A. 2 atoms
Incorrect. In one mole of CH4, there are four moles of hydrogen. The appropriate conversion factors were not utilized.
B. 4 atoms
Incorrect. While there are four atoms in the chemical formula, there are not four atoms of hydrogen in two moles of methane.
C. 1.2 × 1024
Incorrect. This results from the multiplication of two moles of CH4 with 6.02 x 1023. The conversion factor of 1 mol CH4 = 4 mol H was not included.
D. 4.8 x 1024
Correct! Two moles of CH4 contain 4.8 × 1024 atoms of hydrogen.
Calculate the number of molecules in 80.0 grams of water, H2O.
A. 3.34 x 1022 molecules
Incorrect. The student divided 6.02×1023 by the molar mass of the water.
B. 1.87 x 1024 molecules
Incorrect. The student made a calculation error.
C. 2.67 × 1024 molecules
Correct! The student multiplied 6.02×1023 by the mass of water given and divided by the molar mass of water.
D. 4.82 × 1025 molecules
Incorrect. The student forgot to divide by the mass of the water.
The composition of an unknown compound is 50 percent sulfur and 50 percent oxygen. What is the empirical formula of this compound?
A. SO
Incorrect. This formula is inconsistent with the molar ratios.
B. SO2
Correct! The mole ratio of oxygen is twice that of sulfur.
C. SO3
Incorrect. This formula is inconsistent with the molar ratios.
D. S2O3
Incorrect. This formula is inconsistent with the molar ratios.
The empirical formula compound is CH2O (molecular mass = 180 g/mol). What is the molecular formula of the compound?
A. CH2O
Incorrect. Given the molecular mass, this is not the molecular formula.
B. C2H4O2
Incorrect. Dividing the molecular mass by the empirical formula mass does not generate this molecular formula.
C. C4H8O4
Incorrect. Dividing the molecular mass by the empirical formula mass does not generate this molecular formula.
D. C6H12O6
Correct! The empirical formula mass is 30g/mol. Dividing the molecular mass by the empirical formula gives a whole number of six. Multiplying the atoms in the empirical formula by this number results in the molecular formula of C6H12O6.
Aluminum metal reacts with oxygen in the air to form solid aluminum oxide as shown in the equation below.
___Al(s) + ___O2(g) → ___Al2O3(s)
What is the smallest, whole number ratio for Aluminum (Al) once the equation is balanced?
A. 1
Incorrect. The equation will not be balanced.
B. 2
Incorrect. The oxygen on both sides will not be balanced if aluminum has a coefficient of two.
C. 3
Incorrect. A coefficient of three does not balance the aluminum on the reactant side with the aluminum on the product side.
D. 4
Correct! A coefficient of four will balance the equation.
The incomplete chemical equation shown below represents the combustion of propane.
_______ + 5 O2 (g) → 3 CO2 (g) + 4 H2O(g)
What is the missing reactant?
A. C2H8
Incorrect. The number of carbons on the reactant side does not balance the number of carbons on the product side.
B. C2H8O10
Incorrect. The number of carbons on the reactant side does not balance the number of carbons on the product side. Additionally, the total number of oxygen atoms on the reactant side exceeds the number of oxygen atoms on the product side.
C. C3H8
Correct! The formula, C3H8, balances the equation, as there are three carbons and eight hydrogens on the product side.
D. C3H8O10
Incorrect. The total number of oxygen atoms on the reactant side exceeds the number of oxygen atoms on the product side.
The incomplete chemical equation shown below represents the formation of an insoluble solid.
3 AgNO3(aq) + K3PO4(aq) → Ag3PO4(s) + ______
Which of the following best represents the missing product?
A. KNO3
Incorrect. The appropriate chemical formula for the missing reactant is KNO3; however, the number of potassium and nitrate ions is not balanced.
B. 3 KNO3
Correct! The appropriate chemical formula for the missing reactant is KNO3; additionally, a coefficient of three is needed to balance the number of potassium and nitrate ions on the reactant side.
C. K(NO3)3
Incorrect. The appropriate chemical formula for the missing reactant is KNO3, not K(NO3)3; additionally, the number of potassium ions are not balanced.
D. 3 K(NO3)3
Incorrect. The appropriate chemical formula for the missing reactant is KNO3, not K(NO3)3. Additionally, the number of nitrate ions in the product exceeds the number of nitrate ions in the reactant side.
Hydrogen gas and nitrogen gas can be combined industrially to form ammonia. This is reaction is known as the Haber-Bosch process as shown below:
N2 + 3H2 → 2 NH3
How many moles of ammonia could be produced from the reaction of 30 moles of nitrogen and 30 moles of hydrogen?
A. 20 moles NH3
Correct! Hydrogen gas is the limiting reactant and can only produce 20 moles of NH3.
B. 30 moles NH3
Incorrect. The mole ratios are not 1:1 and therefore would not produce 30 moles of NH3.
C. 50 moles NH3
Incorrect. The theoretical yield would not produce 50 moles of NH3 given the mole ratios and the starting quantities of both reactants.
D. 60 moles NH3
Incorrect. Nitrogen gas is the excess reagent while hydrogen gas will produce a smaller mole quantity of NH3.
Consider the following sequential reaction:
ZrC(s) + 4Cl2(g) → ZrCl4(g) + CCl4(g)
ZrCl4(g)+ 2Mg(s)→ Zr(s) + 2MgCl2(l)
How many moles of Zr(s) are produced from 6.00 moles of chlorine gas and excess ZrC(s)?
A. 0.250 mol
Incorrect. This is a sequential reaction. The common link is ZrCl4. To solve the problem, it requires using mole ratios from both equations to solve the problem, starting with Cl2 and ending with Zr.
B. 0.667 mol
Incorrect. This is a sequential reaction. The common link is ZrCl4. To solve the problem, it requires using mole ratios from both equations to solve the problem, starting with Cl2 and ending with Zr.
C. 1.550 mol
Correct! This is a sequential reaction. The common link is ZrCl4. To solve the problem, it requires using mole ratios from both equations to solve the problem, starting with Cl2 and ending with Zr.
6 mol Cl2 × (1 mol ZrCl4/4mol Cl2 ) × (1 mol Zr/1mol ZrCl4)
D. 6.00 mol
Incorrect. This is a sequential reaction. 6.00 moles of chlorine gas will not produce 6.00 moles of ZrC as there is not a 1:1 ratio between the two.