Which of the following statements is true about metallic bonds?
A. Metallic bonds are formed between the atoms of different metals because of the attraction of neutrons and the non-valence electrons.
Incorrect. The neutrons are not attracted to anything, especially inner electrons.
B. Metallic bonds are formed between the atoms of the same metals because of the attraction of the positively charged nucleus and the outer energy electrons.
Correct! The nucleus may not be strong enough to hold on to the electrons in their valence shells, but they are attracted to the sea of electrons.
C. Metallic bonds are formed between the atoms of same metals because of the attraction of neutrons and the outer energy electrons.
Incorrect. The neutrons are not attracted to anything, even outer valence electrons.
D. Metallic bonds are formed between the atoms of different metals because of the attraction of the nucleus and the inner energy electrons.
Incorrect. The nucleuses are attracted to electrons but are holding onto their own core electrons.
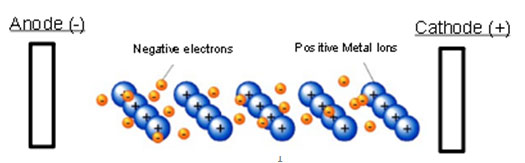
Analyze the diagram above that represents a piece of metal. If an electrical charge was placed on this metal, what would be the most likely outcome at the atomic level?
A. The electrons would stabilize the metal and prevent it from melting.
Incorrect. The metal is not unstable.
B. The metal would get extremely hot and begin to bend.
Incorrect. The metal may not get extremely hot due to an electric charge.
C. The electrons would begin to align with one another and move towards the cathode.
Correct! The electrons would begin to flow towards the cation and create an electric current.
D. The positive metal ions and negative electrons would bond together to create an ionic compound.
Incorrect. Two metals do not create an ionic bond.
Aluminum metal is used for many different purposes, from making soda cans and aluminum siding for manufactured homes, to engine parts and bicycle frames. What property of metals allows aluminum to be used in so many ways?
A. Thermal conductivity
Incorrect. This refers to the ability of metals to transfer heat efficiently.
B. Luster
Incorrect. Luster refers to the sheen of metals.
C. Ductility
Incorrect. Ductility involves stretching into wires.
D. Malleability
Correct! Malleability deals with the ability to bend and deform without breaking.
Metals have many similar properties such as electrical and thermal conductivity, malleability, and ductility. Which of the following statements is a correct summarization of why metals have these properties?
A. The electrons and neutrons are attracted to each other. Neutrons hold the atoms close to each other. This closeness in atoms gives metal their properties.
Incorrect. The electrons are attracted to the positively charged protons.
B. The many valence electrons of metals are shared within the cations. The atoms are gently held together because of the differences in charges of the electrons and the nucleii. This allows electricity and energy to flow and accounts for the metal's ductility and malleability.
Correct! This statement summarizes why metals have properties such as electrical and thermal conductivity, malleability, and ductility.
C. The atoms of metals are tightly packed, which is why heat vibrations will travel so quickly through a sample. Also, the proximity allows for the bending and tensile strength needed for malleability and ductility.
Incorrect. The atoms are tightly packed, but that is not what gives rise to the properties of metals.
D. The valence electrons of one atom are attracted to other valence electrons of another atom. This attraction between valence electrons is the most powerful type of bond.
Incorrect. Why would negative electrons be bonded to each other? They would repel each other.