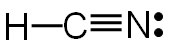
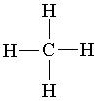
Which of the following is the correct electron pair geometry of CH4?
A. Linear
Incorrect. The structure of CH4 has four electron dense areas attached to the central atom.
B. Tetrahedral
Correct! The structure of CH4 has four
electron dense areas attached to the central atom.
C. Trigonal bipyramidal
Incorrect. The structure of CH4 has four
electron dense areas attached to the central atom.
D. Trigonal planar
Incorrect. The structure of CH4 has four
electron dense areas attached to the central atom.
Which of the following is the correct electron pair geometry of HCN?
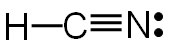
A. Tetrahedral
Incorrect. HCN only has two electron-dense areas around the central atom; therefore, it cannot be tetrahedral in shape.
B. Linear
Correct! HCN has two electron-dense areas around the central atom; therefore, it is linear in shape.
C. Trigonal planar
Incorrect. HCN only has two electron-dense areas around the central atom; therefore, it cannot be trigonal planar in shape.
D. Octaheadral
Incorrect. HCN only has two electron-dense areas around the central atom; therefore, it cannot be octahedral in shape.
What is the correct electron pair geometry of the following molecule?
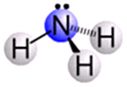
A. Linear
Incorrect. This molecule has four electron-dense areas around the central atom; therefore, it cannot be linear.
B. Trigonal planar
Incorrect. This molecule has four electron-dense areas around the central atom; therefore, it cannot be trigonal planar.
C. Tetrahedral
Correct! This molecule has four electron-dense areas around the central atom; therefore, it has an electron pair geometry of tetrahedral.
D. Trigonal bipyramidal
Incorrect. This molecule has four electron-dense areas around the central atom; therefore, it cannot be trigonal bipyramidal.
A molecule has one lone pair of electrons and two atoms attached to the central atom. Which electron pair geometry would this molecule have?
A. Linear
Incorrect. The molecule described has three electron-dense areas (one lone pair plus two atoms) around the central atom; therefore, it cannot be linear.
B. Trigonal planar
Correct! The molecule has three electron-dense areas around the central atom; therefore, its electron pair geometry is trigonal planar.
C. Tetrahedral
Incorrect. The molecule described has three electron-dense areas (one lone pair plus two atoms) around the central atom; therefore, it cannot be tetrahedral.
D. Trigonal bipyramidal
Incorrect. The molecule described has three electron-dense areas (one lone pair plus two atoms) around the central atom; therefore, it cannot be trigonal bipyramidal.
Which of the following molecules would have the same electron pair geometries?
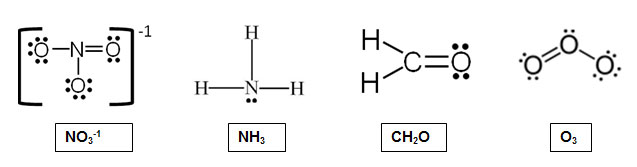
A. NH3, CH2O, O3
Incorrect. O3 and CH2O are trigonal planar, but NH3 is tetrahedral.
B. NO3-1, CH2O
Incorrect. NO3-1 and CH2O are trigonal planar. Is there another molecule that is trigonal planar as well?
C. NH3, NO3-1, CH2O
Incorrect. NO3-1 and CH2O are trigonal planar, but NH3 is tetrahedral.
D. NO3-1, CH2O, O3
Correct! All three of these molecules have the electron pair geometry of trigonal planar.