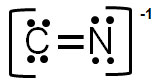
Incorrect. In this structure, the formal charge on carbon would be -2, and the charge on nitrogen would be -1 for an overall charge of -3.
In the molecule SO4-2, which element is the central atom?
A. 0
Incorrect. Oxygen is not the central atom. Which atom is the most electronegative? M
B. S
Correct! Sulfur is the central atom.
C. None
Incorrect. There will always be a central atom.
D. C
Incorrect. Carbon is not a part of the molecule.
Which of the following is the correct electron dot structure of CN-1?
A.
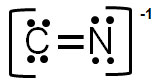
Incorrect. In this structure, the formal charge on carbon would be -2, and the charge on nitrogen would be -1 for an overall charge of -3.
B.
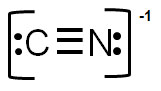
Correct! In this structure, the formal charge on carbon is -1, and the charge on nitrogen is 0 for an overall charge of -1.
C.
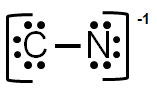
Incorrect. In this structure, the charge on carbon would be -3, and nitrogen is -2 for an overall charge of -5. This structure would have to have 16 electrons when there are 10 available.
D.
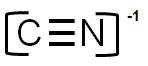
Incorrect. There are not enough electrons shown. There are 10 available and only 6 shown.
When two or more Lewis structures can be used to represent a single molecule, these multiple structures are —
A. polar structures
Incorrect. Polar molecules are when there is a charge differential between the atoms in a molecule.
B. resonance structures
Correct!
C. ionic structures
Incorrect. Ionic molecules are those between metals and nonmetals.
D. isometric structures
Incorrect. This has to do with three-dimensional structures.
How many resonance structures does the molecule NO2-1 have?
A. 1
Incorrect. There is more than one possible.
B. 2
Correct!
C. 3
Incorrect. There are not three possible resonance structures.
D. 4
Incorrect. There are not four possible resonance structures.
Hydrazine is a colorless, flammable liquid and has an odor similar to ammonia. Its chemical formula is N2H4. Which of the following would be the correct Lewis structure?
A.
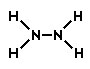
Incorrect. In this structure, the formal charge on each of the nitrogen atoms would be -2, and the charge on each hydrogen atom would be 0. The overall charge of this structure would be -4.
B.
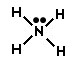
Incorrect. This structure is missing a nitrogen atom.
C.
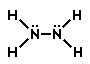
Correct! In this structure, the formal charge on each nitrogen and hydrogen would be zero.
D.
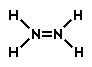
Incorrect. In this structure, the formal charge on each of the nitrogen atoms would be -1 and the charge on each hydrogen atom would be 0. The overall charge of this structure would be -2.