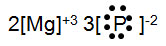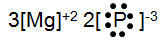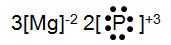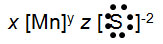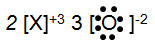Click here to access the Periodic Table of Elements
Magnesium and phosphorous form an ionic bond. Which of the following would be the correct electron for formula to represent this bond?
A.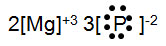
Incorrect. Magnesium is found in group IIA; it has 2 valance electrons that it gives up to phosphorus so that its oxidation number would be +2. Phosphorus is in group VB so it has 5 valance electrons; it needs to gain three electrons to complete the octet. Phosphorus has a oxidation number of -3.
B. 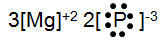
Correct! This is the correct electron for formula.
C.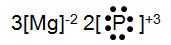
Incorrect. The signs on the oxidation numbers are incorrect. Magnesium is found in group IIA; it has 2 valance electrons that it gives up to phosphorus so that its oxidation number would be +2. Phosphorus is in group VB so it has 5 valance electrons; it needs to gain three electrons to complete the octet. Phosphorus has a oxidation number of -3.
D.
Incorrect. The oxidation numbers are correct, but the oxidation numbers do not add to zero, therefore the compound is not neutral. You need to add numbers in front of the brackets to make the oxidation numbers add to zero.
Click here to access the Periodic Table of Elements
Manganese (Mn) can have several different oxidation numbers. If manganese has an oxidation number of +7 and it bonds with sulfur, what number should be in place x on the electron dot formula below?
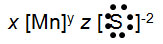
A. +7
Incorrect. The oxidation number is +7; it would go in place of y.
B. 1
Incorrect. If there was only one Mn ion present in the compound, the oxidation numbers would not add to zero and the compound would not be neutral.
C. +3
Incorrect. If there were three Mn ions present in the compound, the oxidation numbers would not add to zero and the compound would not be neutral.
D. 2
Correct! There needs to be two Mn ions in the compound to make the oxidation numbers add to zero.
Click here to access the Periodic Table of Elements
Manganese (Mn) can have several different oxidation numbers. If manganese has an oxidation number of +7 and it bonds with sulfur, what number should be in place z on the electron dot formula below?
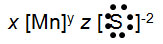
A. 3
Incorrect. If there were three S ions present in the compound, the oxidation numbers would not add to zero and the compound would not be neutral.
B. 1
Incorrect. If there was only one S ion present in the compound, the oxidation numbers would not add to zero and the compound would not be neutral.
C. 7
Correct! There needs to be seven S ions in the compound to make the oxidation numbers add to zero.
D. 2
Incorrect. If there were two S ions present in the compound, the oxidation numbers would not add to zero and the compound would not be neutral.
Click here to access the Periodic Table of Elements
An element, X, reacts to form an ionic compound with oxygen. Below is the electron dot formula for the ionic compound of element X and oxygen. Which answer represents element X?
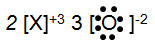
A. Na
Incorrect. Sodium has an oxidation number of +1, not +3.
B. Al
Correct! Aluminum has an oxidation number of 3.
C. K
Incorrect. Potassium has an oxidation number of +1, not +3.
D. Mg
Incorrect. Magnesium has an oxidation number of +2, not +3.
Which of the following diagrams shows the best representation of the interaction between magnesium and bromine atoms in the compound named "magnesium bromide."
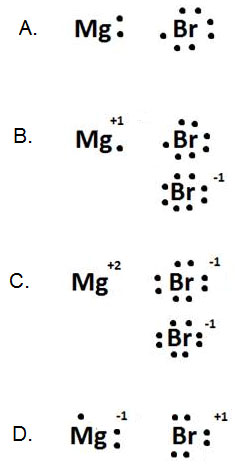
A.
Incorrect. The compound is ionic, so electrons must get transferred.
B.
Incorrect. Although the diagram shows charge-balance, only the bromine ion with a -1 has a complete octet. How can the second bromine atom also achieve this?
C.
Correct! Magnesium transfers two electrons, one to each bromine. The +2 of magnesium is charge-balanced by the two -1 charges of the bromine ions.
D.
Incorrect. Although the diagram shows charge-balance, magnesium is a metal and will lose electrons, while bromine is a nonmetal and will gain electrons to achieve the octet. Think about their placement on the periodic table and the ionic charges they should form.