
Incorrect. This Lewis structure displays two valence electrons. The valence electrons are found in the outermost energy level.
Rutherford’s gold foil experiment consisted of a series of tests that bombarded a thin sheet of gold foil with positively charged alpha particles. Rutherford expected the alpha particles to go straight through the foil. However, he was surprised by the results when he noticed that some alpha particles were deflected at various angles. Which best explains the results of Rutherford’s experiment that eventually led to a change in the atomic structure theory?
A. The gold foil was too large and reflected the alpha particles.
Incorrect. This is not the conclusion that Rutherford and his team came to.
B. Atoms contain positively charged nuclei concentrated in a very small area.
Correct! A few alpha particles were deflected which eventually led to the conclusion that the atom had a positively charged nucleus surrounded mostly by empty space. This led to the downfall of the Plum Pudding Model theory of atomic structure.
C. The gold foil was too thin to allow anything to pass.
Incorrect. The alpha particles passed through the foil; however, a few particles were deflected at various angles.
D. Atoms contain positively charged nuclei concentrated in a very large area.
Incorrect. The nucleus occupies a relatively small space of the atom.
If you were to compare two photons of light, which of the following would be true?
A. A photon with a higher frequency would have a lower energy and a shorter wavelength.
Incorrect. If the energy of a photon decreases, so does its frequency.
B. A photon with a higher frequency would have a higher energy and a longer wavelength.
Incorrect. As the energy increases, so does the frequency. Therefore, the wavelength would not be longer. Additionally, the wavelength would decrease if the frequency increases.
C. A photon with a higher frequency would have lower energy and a longer wavelength.
Incorrect. If the energy of a photon decreases, so does its frequency. Additionally, the wavelength would decrease if the frequency increases.
D. A photon with a higher frequency would have higher energy and a shorter wavelength.
Correct! As the energy increases, so does the frequency. Additionally, this would require that the wavelength be shorter as a result.
Which of the following best describes the relationship between wavelength and wave energy in the electromagnetic spectrum?
A. Wave energy decreases as the wavelength increases.
Incorrect. This does not demonstrate the mathematical relationship between energy and wavelength.
B. Wave energy increases as the wavelength decreases.
Correct! As the energy of a wave increases, the wavelength decreases.
C. Wave energy is unpredictable when wavelength is constant.
Incorrect. This does not demonstrate the mathematical relationship between energy and wavelength.
D. Wave energy is constant regardless of changes in wavelength.
Incorrect. This does not demonstrate the mathematical relationship between energy and wavelength.
Which of the following best compares a photon with a wavelength of 260nm and a photon with a wavelength of 340nm?
A. A 340nm photon has a longer wavelength, lower frequency, and lower wave energy.
Correct! Longer wavelength would indicate a lower frequency, in addition to a lower wave energy.
B. A 340nm photon has a longer wavelength, higher frequency, and incorrect wave energy.
Incorrect. This does not correctly compare the two wavelengths.
C. A 340nm photon has a shorter wavelength, lower frequency, and lower wave energy.
Incorrect. This does not correctly compare the two wavelengths.
D. A 340nm photon has a shorter wavelength, higher frequency, and lower wave energy.
Incorrect. This does not correctly compare the two wavelengths.
Which of the following best describes the mathematical relationship between the electromagnetic radiation and wavelength?
A. The lower the energy of electromagnetic radiation, the shorter its wavelength.
Incorrect. Lower energy would result in a longer wavelength based on the mathematical relationship between the electromagnetic radiation and wavelength.
B. The higher the energy of electromagnetic radiation, the shorter its wavelength.
Correct! As the energy of electromagnetic radiation increase, the frequency will increase. As a result, the wavelength will decrease.
C. The lower the energy of electromagnetic radiation, the wavelength becomes constant.
Incorrect. Wavelength does not become constant as a result of the low electromagnetic radiation energy.
D. The higher the energy of electromagnetic radiation, the wavelength varies greatly.
Incorrect. Wavelength does not vary greatly as a result of the high electromagnetic radiation energy.
Calculate the energy of a photon whose frequency is 3.17 × 1017 Hz.
A. 4.79 × 1015 J
Incorrect. This is not the energy of a photon for this problem.
B. 2.10 × 10-16 J
Correct! The energy of a photon for this problem is determined by multiplying the frequency by Planck’s constant.
C. 2.09 × 10-51 J
Incorrect. The energy of a photon for this problem is not determined by dividing Planck’s constant by the frequency.
D. 4.78 × 1050 J
Incorrect. The energy of a photon for this problem is not determined by dividing the frequency by Planck’s constant.
When atoms are excited, light is released in tiny packets called photons. Sodium-vapor lamps, also known as streetlights, use sodium in an excited state to produce yellow light. Calculate the frequency of yellow light whose energy is 3.37 × 10-19 joules.
A. 1.97 × 10-15 Hz
Incorrect. The frequency is not calculated by dividing Planck’s constant by the energy.
B. 3.37 × 10-19 Hz
Incorrect. This is the energy of the photon.
C. 5.09 × 1014 Hz
Correct! Frequency is calculated by dividing the energy by Planck’s constant.
D. 5.9 × 10-7 Hz
Incorrect. This is calculated wavelength, not the frequency.
What is the energy of a wave with a frequency of 5.0 × 107Hz?
A. 1.33 × 10-41 J
Incorrect. The energy of the wave is not the quotient of Planck’s constant and frequency.
B. 1.50 × 1016 J
Incorrect. The energy of the wave is not calculated using Avogadro’s number.
C. 3.31 × 10-26 J
Correct! The energy of the wave is the product of Planck’s constant and frequency.
D. 7.00 × 1040 J
Incorrect. The energy of the wave is not the quotient of frequency and Planck’s constant.
Rubidium has two common isotopes: 85Rb and 87Rb. What is the calculated average atomic mass of rubidium if 72.2% of rubidium is 85Rb and 27.8% is 87Rb?
A. 27.80 amu
Incorrect. This is not the average atomic mass of rubidium.
B. 35.37 amu
Incorrect. This is not the average atomic mass of rubidium.
C. 72.20 amu
Incorrect. This is not the average atomic mass of rubidium.
D. 85.56 amu
Correct! This is the calculated average atomic mass of rubidium.
Element X has two common isotopes, as shown in the table below.
|
Isotope
|
Percent Abundance
|
|
63X
|
69.08%
|
|
65X
|
30.91%
|
What is the identity of element X?
A. Nickel
Incorrect. The calculated average atomic mass does not reflect the atomic mass of nickel.
B. Copper
Correct! Using only the atomic masses provided in the nuclide symbol, the closest calculated atomic mass is copper.
C. Zinc
Incorrect. The calculated average atomic mass does not reflect the atomic mass of zinc.
D. Gallium
Incorrect. The calculated average atomic mass does not reflect the atomic mass of gallium.
Element Z has two common isotopes, as shown in the table below.
|
Isotopic Mass
|
Percent Abundance
|
|
10.0
|
19.7%
|
|
11.0
|
80.2%
|
What is the identity of element Z?
A. Beryllium
Incorrect. The calculated average atomic mass does not reflect the atomic mass of beryllium.
B. Boron
Correct! The closest calculated atomic mass is boron. (10.0×0.197)+(11.0×0.802)
C. Fluorine
Incorrect. The calculated average atomic mass does not reflect the atomic mass of fluorine.
D. Bromine
Incorrect. The calculated average atomic mass does not reflect the atomic mass of bromine.
The isotopic information for element M is shown in the table below.
|
Isotope
|
Percent Abundance
|
|
39M
|
78.8&
|
|
40M
|
18.1%
|
|
41M
|
3.1%
|
Which of following is the calculated atomic mass of element M?
A. 39.2 amu
Correct! This is the calculated average atomic mass of element M.
(39×0.788)+(40×0.181)+(41×.031)
B. 40.0 amu
Incorrect. This is not the calculated average atomic mass of element M.
C. 41.2 amu
Incorrect. This is not the calculated average atomic mass of element M.
D. 42.0 amu
Incorrect. This is not the calculated average atomic mass of element M.
Which of the following properly expresses the electron configuration of sulfur (S)?
A. 1s21p62s22p6
Incorrect. Sulfur’s valence shell is in the third energy level. Additionally, energy level one does not contain p-orbitals.
B. 1s22s22p6 3s23p4
Correct! Sulfur’s valence shell is in the third energy level. Additionally, it has six valence electrons as show in the s and p-orbitals.
C. 1s22s22p33p33d4
Incorrect. Sulfur does not contain d-orbital electrons. Additionally, the 2p-orbital is partially filled.
D. 1s22s22p33s23p63d1
Incorrect. Sulfur does not contain d-orbital electrons. Additionally, the 2p-orbital is partially filled.
Which of the following would best express the outer electron configuration of a noble gas?
A. ns2np5
Incorrect. This notation indicates that the unknown atom has seven valence electrons. Additionally, the p-orbitals are not filled.
B. ns2np6
Correct! This notation indicates that the unknown atom has eight valence electrons. Additionally, the s and p-orbitals are appropriately filled.
C. ns2np7
Incorrect. The six electrons may occupy the p-orbitals. This notation indicates that there are seven electrons in the p-orbital.
D. ns2np8nd1
Incorrect. The six electrons may occupy the p-orbitals. This notation indicates that there are eight electrons in the p-orbital.
Which of the following best expresses the Lewis valence electron dot structure for an atom with the electron configuration of 1s22s22p63s23p1?
A.

Incorrect. This Lewis structure displays two valence electrons. The valence electrons are found in the outermost energy level.
B.

Correct! The electron configuration shows that the atom has three electrons in its valence shell as indicated by the 3s and 3p orbitals. This Lewis structure displays three valence electrons.
C.

Incorrect. This Lewis structure displays six valence electrons. The valence electrons are found in the outermost energy level.
D.

Incorrect. This Lewis structure displays seven valence electrons. The valence electrons are found in the outermost energy level.
An atom has the following Lewis valence electron dot structure.

Which of the following electron configurations would best represent the structure shown above?
A. 1s21p5
Incorrect. The first energy level does not have a p-orbital.
B. 1s22s32p2
Incorrect. The s-orbital can hold a maximum of two electrons.
C. 1s22s22p3
Correct! The Lewis structure shown in the problem has five valence electrons. This is indicated in the 2s and 2p orbitals.
D. 1s22s22p63s2
Incorrect. The Lewis structure shown in the problem has five valence electrons.
Which of the following best describes the type of radioactive decay shown below?
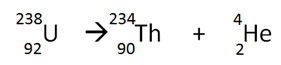
A. Alpha decay
Correct! The nuclear equation above illustrates decay of 238U into 234Th through the reduction of both the atomic mass and number. This is characteristic of alpha decay reactions.
B. Beta decay
Incorrect. This is not a loss of electrons shown in the nuclear equation to indicate beta decay.
C. Gamma decay
Incorrect. The nuclear equation shows a change in the chemical element which is not characteristic of gamma decay reactions.
D. Delta decay
Incorrect. This term does not address the radioactive decay process shown.
Some atomic nuclei undergo changes by emitting a stream of high-energy photons, which results in a loss of energy. The process does not result in a loss of protons or neutrons for an element and the parent and daughter atoms are the same chemical element. Which of the following best characterizes this decay process?
A. Alpha decay
Incorrect. Alpha decay results in a reduction in atomic mass and number.
B. Beta decay
Incorrect. Beta decay results in the nucleus of an atom changing into the nucleus with one higher atomic number.
C. Gamma decay
Correct! This describes a characteristic of gamma decay.
D. Delta decay
Incorrect. This term does not address this decay process.
Which of the following best describes the type of radioactive decay shown below?
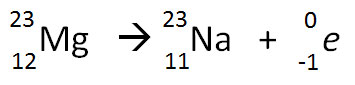
A. Alpha decay
Incorrect. The nuclear equation above illustrates the loss of electrons, which is not characteristic of alpha decay.
B. Beta decay
Correct! This shows the loss of electrons in the nuclear equation to indicate beta decay.
C. Gamma decay
Incorrect. The nuclear equation above illustrates the loss of electrons and change of chemical element, which is not characteristic of gamma decay.
D. Delta decay
Incorrect. This term does not address the radioactive decay process shown.
The nuclear equation below shows the beta decay of 9Li.
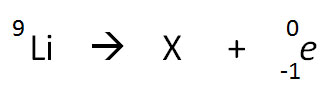
Which of the following nuclide symbols would best represent X in order to balance the nuclear equation?
A. 9Li
Incorrect. The nuclear equation depicted is an example of a beta decay process with the loss of an electron, not a positron. Therefore, the nucleus would not be changed into a nucleus with a lower atomic number.
B. 9Be
Correct! The nuclear equation depicted is an example of a beta decay process, with the loss of an electron. The nucleus of an atom is changed into the nucleus with one higher atomic number – in this case, Beryllium.
C. 9F
Incorrect. The nuclear equation depicted is an example of a beta decay process with the loss of an electron. While the Lithium nucleus would be changed, it would only change into a nucleus with one higher atomic number than itself, which would not be fluorine.
D. 0N
Incorrect. The nuclear equation depicted is an example of a beta decay process. This would not be the appropriate nuclide symbol.
The nuclear equation below shows the alpha decay of 218Po.
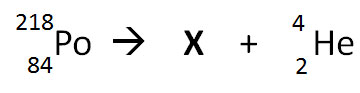
Which of the following nuclide symbols would best represent X in order to balance the nuclear equation?
A.
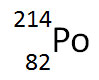
Incorrect. Alpha decay results in the decrease of the atomic mass and a decrease in atomic number resulting in a change of the chemical element.
B.
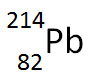
Correct! Alpha decay results in the decrease of the atomic mass by four and a decrease in atomic number by two.
C.
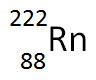
Incorrect. Alpha decay results in the decrease of the atomic mass and a decrease in atomic number.
D.
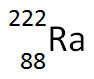
Incorrect. Alpha decay results in the decrease of the atomic mass and a decrease in atomic number.
The nuclear equation below shows the decay of 238U.
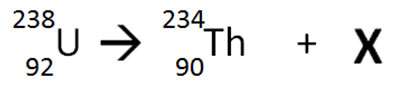
Which of the following particles would best represent X in order to balance the nuclear equation?
A.
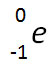
Incorrect. The decay of 238U into 234Th is not the result of a loss of an electron and does not balance the nuclear equation.
B.
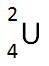
Incorrect. This nuclide symbol does not balance the nuclear equation.
C.
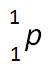
Incorrect. This nuclide symbol does not balance the nuclear equation.
D.
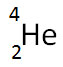
Correct! The decay of 238U into 234Th is the result in the decrease of atomic number (by 2) and atomic mass (by 4) by the loss of an alpha particle.
Which of the following describes how fusion reactions differ from fission reactions?
A. Fusion reactions result in the splitting of the atomic nucleus.
Incorrect. Fission reactions result due to the splitting of the atomic nucleus.
B. Fusion reactions result by combining light nuclei together.
Correct! Fusion reactions are the result of the combination of two light nuclei.
C. Fusion reactions result in the production of heat.
Incorrect. Both fusion and fission reactions generate heat.
D. Fusion reactions result in a series of chain reactions.
Incorrect. Fusion reactions do not involve chain reactions, unlike fission reactions.
Which of the following best describes why fusion reactions are difficult to begin?
A. Fusion reactions require the combination of two positively charge nuclei, which repel each other.
Correct! Fusion requires combining two nuclei, which are positively charge and like charges repel each other.
B. Fusion reactions require energy to escape the attraction of the nucleus.
Incorrect. Fusion requires combining two nuclei. The positively charge nuclei of two atoms are not attracted to each other; they repel each other.
C. Fusion reactions require the combination of two electrons, which repel each other.
Incorrect. Fusion requires combining two nuclei, which are positively charge.
D. Fusion reactions require chain reactions that are applied to several atoms at once.
Incorrect. Fusion reactions do not require chain reactions to take place.
Which of the following reactions occurs as a result of a neutron bombarding a heavy nucleus?
A. Alpha decay
Incorrect. Alpha decay occurs due to the emission of an alpha particle, which results in the decrease in atomic number and atomic mass.
B. Fusion
Incorrect. Fusion occurs as a result of two nuclei coming together.
C. Beta decay
Incorrect. Beta decay results in the loss of an electron or gain of a positron.
D. Fission
Correct! Fission occurs due to the collision of the nucleus with a neutron.