
A. Bi-205
Incorrect. Since the isotope loses 2 protons it has a new atomic number and thus becomes a different element.
B. Tl-207
Incorrect. The atomic mass is wrong here.
C. At-211
Incorrect. It does not gain particles.
D. Tl-205
Correct! Bi 209 loses 2 protons and 2 neutrons.
Thorium-229 helps fluorescent lights last longer. What type of radioactive decay is indicated by the following transmutation equation?

A. Alpha decay
Correct! Th 229 loses 2 neutrons and 2 electrons.
B. Beta decay
Incorrect. Th 229 loses 2 neutrons and 2 protons.
C. Gamma decay
Incorrect. Th 229 loses 2 neutrons and 2 protons.
D. Electron capture
Incorrect. Th 229 loses 2 neutrons and 2 protons.
Iodine-134 has a half-life of 52 minutes, which makes it useful as a radioactive tracer in medical testing. What decay particle is missing in the following decay equation for this isotope?
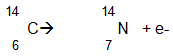
A. Alpha decay
Incorrect. There is a gain of a proton.
B. Beta decay
Correct! There is an emission of an electron.
C. Gamma decay
Incorrect. This equation does not indicate gamma decay.
D. There is no decay.
Incorrect. There is the emission of an electron.
A small amount of cobalt is an essential requirement for most living organisms. In human beings, it is one of the key components of vitamin B12. What are the missing components of the following decay reaction?

A. Alpha decay
Incorrect. There is a gain of a proton.
B. Beta decay
Correct! There is a gain of a proton.
C. Gamma decay
Incorrect. Although there may be some gamma decay, it is not indicated in the equation.
D. No decay
Incorrect. There is a gain of a proton.
The following products were found after a mineral was placed on a photo-reactive plate. What is the most probable starting isotope for these products?
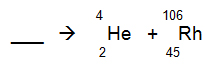

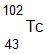
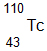

A.
Correct! The isotope loses 2 protons and 2 neutrons.
B.
Incorrect. The isotope loses 2 protons and 2 neutrons.
C.
Incorrect. The isotope loses 2 protons and 2 neutrons.
D.
Incorrect. The isotope loses 2 protons and 2 neutrons.