A hydrogen atom is in a ground state when its electron —
A. has moved away from the atom to the ground
Incorrect. This is not an accurate description of ground state.
B. has stopped moving
Incorrect. This is not an accurate description of ground state.
C. is moving back and forth through the nucleus
Incorrect. This is not an accurate description of ground state.
D. remains at the lowest energy level
Correct! This is the correct description of ground state.
According to Bohr’s model of the atoms, an atom of hydrogen emits light when its electron —
A. moves from a lower-energy level to a higher-energy level
Incorrect. When an electron jumps from a lower energy level to a higher one it absorbs energy.
B. moves from a higher-energy level to a lower-energy level
Correct! When an electron falls from a higher energy level to a lower energy level it releases energy and light is emitted.
C. is in the ground state
Incorrect. According to Bohr’s model, electrons in the ground state do not emit or absorb energy.
D. collides with the nucleus
Incorrect. Electrons do not collide with the nucleus in the Bohr model.
One limitation of Bohr’s model of the atom is that it did not explain —
A. why electrons in the ground state do not emit electromagnetic energy
Correct! Bohr’s model does not explain why electrons in the ground state do not emit electromagnetic radiation.
B. the absorption spectrum of hydrogen
Incorrect. Bohr’s model does explain the absorption spectrum of hydrogen.
C. the emission spectrum of hydrogen
Incorrect. Bohr’s model does explain the emission spectrum of hydrogen.
D. that there are no limitations to Bohr's model of the atom
Incorrect. There are limitations to Bohr’s model of the atom.
Click on this link to access the Chemistry STAAR resource sheet.
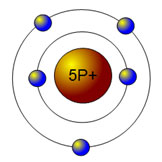
Which atom is represented by the picture below?

A. Beryllium
Incorrect. Beryllium has 4 electrons and 4 protons.
B. Boron
Correct! Boron has 5 electrons and 5 protons.
C. Lithium
Incorrect. Lithium has 3 electrons and 3 protons.
D. Nitrogen
Incorrect. Nitrogen has 7electrons and 7 protons.
Click on this link to access the Chemistry STAAR resource sheet.
How many electrons are in the third energy level of Phosphorous (P)?
A. 10
Incorrect. Phosphorous has 15 electrons total. The first energy level would have 2 and the second would have 8. The remaining 5 would be in the third energy level.
B. 3
Incorrect. That would not be enough electrons.
C. 5
Correct! Phosphorous has 15 electrons total. The first energy level would have 2 and the second would have 8. The remaining 5 would be in the third energy level.
D. 6
Incorrect. That would be too many electrons.