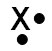
Incorrect. If this element has six protons, then the element is carbon. Carbon has six electrons but only four valence electrons.
Element X contains six protons, six electrons, and six neutrons. Which Lewis Dot diagram below represents element X? Use the STAAR Reference Material if needed.
A.
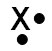
Incorrect. If this element has six protons, then the element is carbon. Carbon has six electrons but only four valence electrons.
B.

Correct! If this element has six protons, then the element is carbon. Carbon has six electrons but only four valence electrons.
C. 
Incorrect. If this element has six protons, then the element is carbon. Carbon has six electrons but only four valence electrons.
D.

Incorrect. If this element has six protons, then the element is carbon. Carbon has six electrons but only four valence electrons.
Two elements would have the same Lewis valence electron dot structures (the chemical symbol would be unique) if they —
A. are in the same row on the periodic table
Incorrect. Rows on the periodic table have the same number of energy levels.
B. are in the same column on the periodic table
Correct! All the elements in the same column on the periodic table have the same number of valence electrons.
C. have the same atomic mass
Incorrect. Atomic mass has no bearing on the Lewis valence electron dot structures.
D. have the same atomic number
Incorrect. Each element has a unique atomic number.
What information can a Lewis valence electron dot structure tell you?
A. The number of electrons in the outermost energy level
Correct! Only the valence electrons are shown in Lewis valence electron dot structures.
B. The total number of electrons
Incorrect. Only the valence electrons are shown in Lewis valence electron dot structures.
C. The number of neutrons
Incorrect. Neutrons are not shown in Lewis valence electron dot structures.
D. The period the element belongs to
Incorrect. The group the element belongs to can be determined from the Lewis valence electron dot structure but not the period.
Which of the following Lewis valence electron dot structures is correct? Use the STAAR Reference Material if needed.
A.
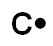
Incorrect. Carbon would have four valence electrons.
B.
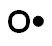
Incorrect. Oxygen has six valence electrons.
C.
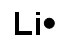
Correct! Lithium has one valence electron.
D.

Incorrect. Neon has eight valence electrons.