Based on the periodic trends for atomic radii, which of the following elements is expected to have the largest atomic radius?
Click here to access the STAAR resource page.
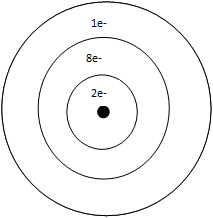
A. Aluminum
Incorrect. Aluminum is in period 3.
B. Cesium
Correct! Cesium is in period 6 and only has 1 valence electron.
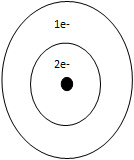
C. Helium
Incorrect. Helium is in period 1.
D. Radon
Incorrect. Radon is in period 6, but has more valence electrons than cesium.
Which of the following lists the elements in decreasing electronegativity?
Click here to access the STAAR resource page.
A. calcium, sodium, sulfur, fluorine
Incorrect. Fluorine is the most electronegative.
B. sodium, calcium, sulfur, fluorine
Incorrect. Fluorine is the most electronegative.
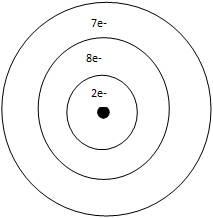
C. fluorine, calcium, sulfur, sodium
Incorrect. Sulfur is more electronegative than calcium.
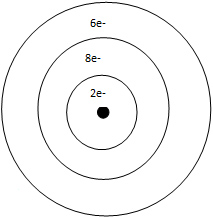
D. fluorine, sulfur, calcium, sodium
Correct! Fluorine is the most electronegative, and sodium is the least electronegative.
Click here to access the STAAR resource page.
Which of these elements would have the lowest ionization energy?
A. |
B. |
C. |
D. |
A.
Incorrect. Lithium has a higher ionization energy because the lone electron is closer to the nucleus.
B.
Correct! Sodium has the lowest ionization energy because it takes less energy to remove the lone electron in the outer shell.
C.
Incorrect. Sulfur has a higher ionization energy because it takes more energy to remove an electron when there are multiple electrons in the outer shell.
D.
Incorrect. Chlorine has a higher ionization energy because it takes more energy to remove an electron when there are multiple electrons in the outer shell.
Click here to access the STAAR resource page.
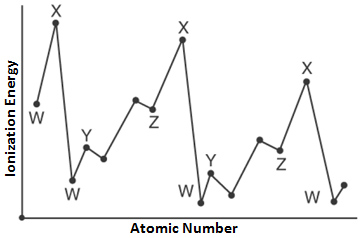
The chart above shows the relationship between the atomic number of an element and its first ionization energy. The letter on the chart that represents the ionization energy of noble gases is —
A. W
Incorrect. W represents the alkali metal family.
B. X
Correct! X represents the noble gases because they that have electrons that are the hardest to remove.
C. Y
Incorrect. Y represents alkaline earth metals.
D. Z
Incorrect. Z represents the halogens.
Click here to access the STAAR resource page.
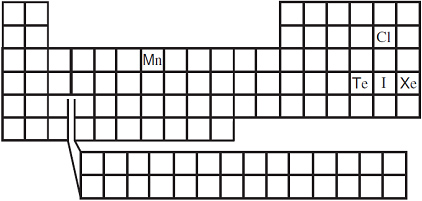
Which of the following elements shown on the Periodic Table above has the highest electronegativity?
A. Cl
Correct! The most electronegative elements are on the upper right-hand side of the periodic table.
B. I
Incorrect. Iodine is lower on the periodic table than chlorine.
C. Mn
Incorrect. Manganese is lower and farther to the left side on the periodic table than chlorine.
D. Xe
Incorrect. Tellurium is lower on the periodic table than chlorine.