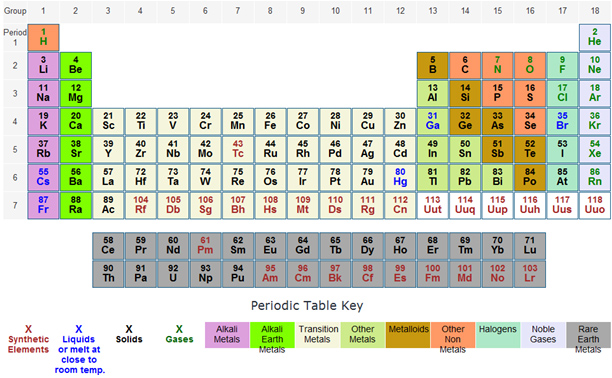

Which of the following collections of elements has the most similar chemical properties?
A. Ca, Ti, Fe
Incorrect. Elements in the same group, not the same period have similar chemical properties.
B. C, Si, Pb
Correct! Elements in the same group have similar chemical properties.
C. B, C, N
Incorrect. Elements in the same group, not the same period have similar chemical properties.
D. K, Mg, Al
Incorrect. Elements in the same group, not the same period have similar chemical properties.

A student hypothesizes that Potassium (K) and Calcium (Ca) have different chemical properties. The periodic table supports this hypothesis by demonstrating that —
A. Calcium and potassium are both metals.
Incorrect. Determination of similar or different chemical properties is based on the number of electrons in the outer energy shells, not classification (i.e. metal, non-metal, etc.)
B. Calcium and potassium are in the same family.
Incorrect. Calcium and potassium are not in the same family. They are in the same period (horizontal row).
C. Calcium and potassium have a different number of valence electrons.
Correct! Elements within in the same group/family have similar chemical properties due to the fact that they have the same number of valence electrons.
D. Calcium is more dense than potassium.
Incorrect. Determination of similar or different chemical properties is based on the number of electrons in the outer energy shells, not physical properties.

A student was given an element sample and then was told to try and identify the mystery element. The student determined that the element had properties similar to copper and gold. Which of the following is most likely to be the mystery element?
A. Ni (nickel)
Incorrect. Elements in the same group/family have similar properties. Ni is not in the same group/family as Cu and Au.
B. Pt (platinum)
Incorrect. Elements in the same group/family have similar properties. Ni is not in the same group/family as Cu and Au.
C. Sn (tin)
Incorrect. Elements in the same group/family have similar properties. Ni is not in the same group/family as Cu and Au.
D. Ag (silver)
Correct! Ag (silver) is located in the same family/group as Cu (copper) and gold (Au).

Which of the following groups of elements has the least similar chemical properties?
A. Hydrogen (H), Radon(Rn), Zinc(Zn)
Correct! None of these elements share a group/family. They are not in adjacent groups/families either.
B. Lithium(Li), Potassium(K), Cesium(Cs)
Incorrect. These elements all share Group I.
C. Cobalt (Co), Nickel(Ni), Platinum (Pt)
Incorrect. These three elements are in groups adjacent to one another. They are Transition Metals.
D. Helium (He), Argon(Ar), Krypton(Kr)
Incorrect. These elements are all Noble Gasses.