A liquid is a state of matter that —
A. flows
Correct! A liquid's molecules, as well as a gas's have weak attractive forces which allow them to move past one another.
B. has a definite shape
Incorrect. A liquid will take the shape of the container it is in due to the nature of its attractive forces.
C. has no definite volume
Incorrect. Liquids have a definite volume because they are not compressible.
D. is easily compressed
Incorrect. Liquids cannot be compressed due to their density.
A gas is a state of matter that —
A. has a definite volume
Incorrect. The lack of strong attractive forces allows a gas to expand to fill its container, therefore assuming the container's volume.
B. is difficult to compress
Incorrect. Because gas molecules are widely separated with weak attractive forces, they can be easily compressed.
C. is generally a liquid or solid at room temperature
Incorrect. This cannot be determined in a general statement. The state at room temperature is specific to a particular substance.
D. takes the shape and volume of its container
Correct! The lack of strong attractive forces between gas molecules allows it to expand to fill its container, taking on the container's shape and volume.
A solid is a state of matter that —
A. has its particles tightly packed together
Correct! The strong attractive forces between a solid's molecules are strong enough to hold them close together and lock them in place, relative to one another.
B. is easily compressed
Incorrect. Solids are not very compressible because the molecules have little free space tetween them.
C. takes the shape and volume of its container
Incorrect. Solids have their own shape and volume because their molecules are held in a fixed position.
D. takes the shape of its container
Incorrect. Solids have their own shape because their molecules are held in a fixed position.
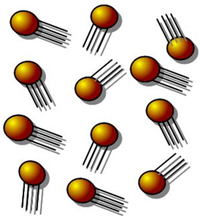
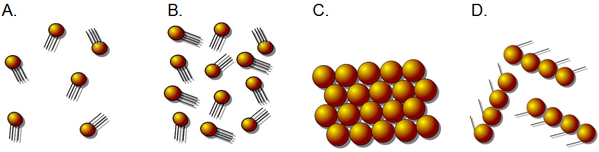
The diagram above shows a model of gas particles at room temperature.
Which of the following diagrams best shows the results of removing heat from these gas particles until they are frozen?

A.
Incorrect. This diagram shows the model above, but with some of the particles.
B.
Incorrect. This diagram is the exact same one as the model above.
C.
Correct! This diagram shows the particles in a tightly-packed, orderly arrangement as is typical of solids.
D.
Incorrect. This diagram shows a random grouping of particles which does not depict any of the 3 states of matter.
When pressure and temperature are constant, a liquid is different from a gas because the molecules of the liquid —
A. are in constant motion
Incorrect. Molecules of all states of matter, solids, liquids, and gases, are in constant motion.
B. have no regular arrangement
Incorrect. Molecules in both liquids and gases have no regular arrangement, whereas solids do.
C. have stronger forces of attraction between them
Correct! The molecules of a liquid are closer together and in constant contact, whereas in a gas the molecules are widely spaced and only come in contact through collisions.
D. take the shape of the container they are in
Incorrect. Molecules of both liquids and gases take the shape of the container they are in.