Click here to access the Periodic Table in the STAAR resources.
According to the Periodic Table, the chemical oxygen, O, will most likely form an ionic bond with —
A. Carbon, C
Incorrect. This is a covalent bond not an ionic one; it has two nonmetals.
B. Magnesium, Mg
Correct! Magnesium has two electrons in its valence shell that can donate to oxygen. Mg becomes a cation and O becomes an anion causing an electrostatic attraction and an ionic bond.
C. Sulfur, S
Incorrect. This is a covalent bond where both elements in the compound want to gain electrons.
D. Tin, Sn
Incorrect. Tin has a higher electronegativity and ionization energy than Mg so that the element would be more likely to bond.
Click here to access the Periodic Table in the STAAR resources.
What type of bonding is associated with compounds that have the following characteristics:
A. Covalent
Incorrect. Covalent compounds have low melting points and are poor electrical conductors.
B. Hydrogen
Incorrect. Hydrogen bonds often cause compounds to have low melting points.
C. Ionic
Correct! Ionic bonded compounds have each of the characteristics listed.
D. Metallic
Incorrect. Metallic bonded compounds are not usually soluble in a solution.
Covalent bonds usually occur between —
A. a nonmetallic element and a metallic element
Incorrect. These form ionic bonds.
B. a metalloid and a metallic element
Incorrect. These form ionic bonds.
C. two metallic elements
Incorrect. These form metallic bonds.
D. two nonmetallic elements
Correct! These form covalent bonds.

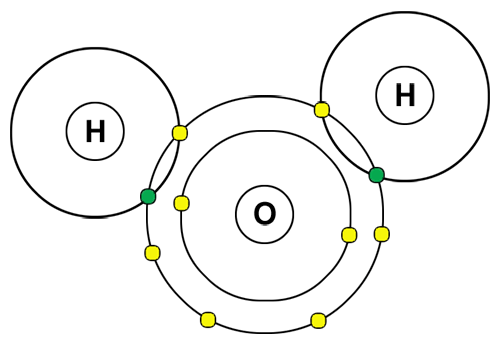
Examine the diagram above. Based upon the electrons' locations in the energy levels, which type of bond is being shown in the water molecule above?
A. Covalent
Correct! The hydrogen and oxygen atoms are sharing electrons.
B. Ionic
Incorrect. The hydrogen and oxygen atoms have a strong bond as they are sharing electrons.
C. Hydrogen
Incorrect. The hydrogen and oxygen atoms have a strong bond as they are sharing electrons.
D. Metallic
Incorrect. The hydrogen and oxygen atoms are not metals.
Click here to access the Periodic Table in the STAAR resources.
Which compound has both ionic and covalent bonding?
A. CH2Cl
Incorrect. The C-H and C-Cl bonds are both covalent.
B. C2H5OH
Incorrect. The C-H, C-C, C-O, and O-H bonds are all covalent.
C. CO2
Incorrect. The C=O bond is covalent.
D. Na3PO4
Correct! The P-O and P=O are bonds that are both covalent, the Na-O bond is ionic.
Looking at the table below, which of the following combinations would create an ionic compound?
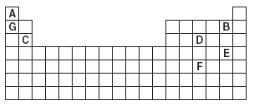
A. A and E
Correct! This is a bond from a metal cation and a nonmetal anion.
B. D and B
Incorrect. This would be a covalent compound.
C. D and D
Incorrect. This would be a covalent compound.
D. C and C
Incorrect. These are two metals that would create an alloy.