This sphere represents one atom of oxygen.

This is three atoms of oxygen.

This image represents six atoms. There are three different elements, oxygen, carbon and helium, represented.
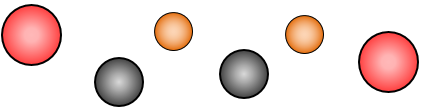
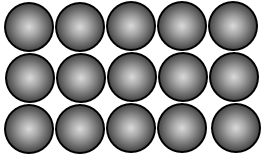
This image represents many atoms in a solid. Notice the atoms are not joined or bonded to each other they are just packed very tightly together.
An element is made up of only one kind of atom. Chemical symbols are used to represent each element. Elements can be found on the Periodic Table of Elements.
Every element has a unique set of properties that can be used to identify the element. These characteristics are the same no matter what amount of the element is present. These properties include boiling point, melting point, density, and reactivity.
Look at the elements in the table below. You are probably familiar with these elements. These three elements have some similar properties, but each element can be identified by its unique set of properties.
| Copper | Silver | Gold |
 |
 |
 |
|
|
|
A molecule is 2 or more atoms joined together by a chemical bond.
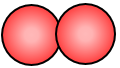
Since these atoms are the same, it is also an element.
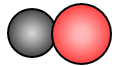
In this molecule, there are two different atoms joined together. A compound is a pure substance made up of two or more elements that are chemically combined. This compound is made up of one atom of carbon and one atom of oxygen.
Molecules are represented by chemical formulas. Chemical formulas contain the chemical symbols of the elements present in the molecule as well as subscripts that indicate the number of each type of atom.

In this molecule, there are two atoms of oxygen. The chemical formula would be O2.

Carbon monoxide is a compound. One carbon monoxide molecule is made up of one atom of carbon and one atom of oxygen. The chemical formula for carbon monoxide is CO. When there is just one atom of the element present a subscript is not used.

Carbon dioxide is a compound. One carbon dioxide molecule is made up of one atom of carbon and two atoms of oxygen. The formula for carbon dioxide is CO2.
A compound has different properties from the elements that form it. For example, the compound sugar contains the elements oxygen, carbon, and hydrogen. The chart below shows the properties of oxygen, carbon, and hydrogen.
| Oxygen | Carbon | Hydrogen |
|
|
|

When these elements combine in the appropriate ratios to form the compound sugar, their properties are not the same at all. The properties of sugar are a sweet, white, crystalline solid. A totally new substance is created even though it contains the elements carbon, hydrogen, and oxygen.
The air that we breathe is a mixture. It is made up of many different elements and compounds.

Mixtures are composed of two or more substances that each keep their original properties and do not combine chemically when put together. A salad is an example of a mixture. Each part keeps its properties—lettuce, tomatoes, carrots, all taste like themselves--but they are all combined. The different parts of a mixture can be separated by physical forces.
Air contains different elements and compounds, therefore; it is considered a mixture. Each element or compound in the mixture still maintains its properties. The oxygen in the air is mixed with other elements and compounds, but it is still oxygen.