Source: VSEPR Theory and molecular shapes, IB Chemistry Blog
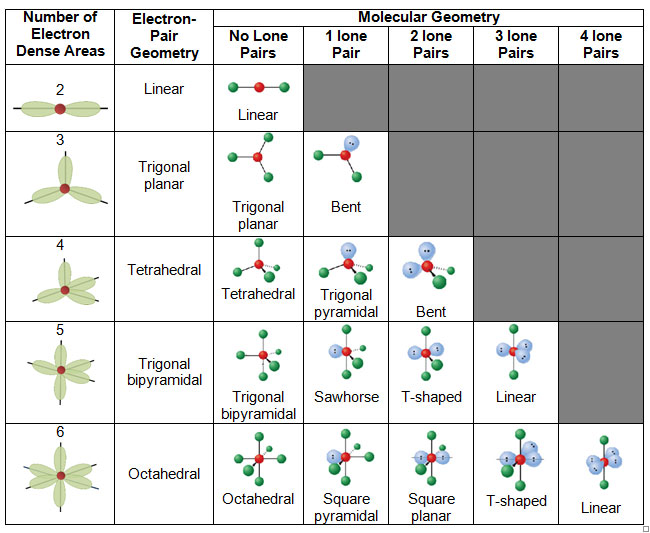
When determining the molecular geometry, only the bonded atoms, not the lone pairs, are considered. In simple terms, the unbound lone pairs are not always drawn, but they still affect the shape of the molecule due to their repulsive properties. If a molecule contains atoms only bonded to the central atom and no lone pairs present, the molecular geometry and the electron-pair geometry are the same.
The table below shows the electron-pair and molecular geometries.

Source: VSEPR Theory and molecular shapes, IB Chemistry Blog
![]() Watch this video to learn more about molecular geometry and how it compares to electron-pair geometry.
Watch this video to learn more about molecular geometry and how it compares to electron-pair geometry.
Source: Molecular Geometry VS Electron Geometry - The Effect of Lone Pairs on Molecular Shape, ChemAssistBeta,YouTube
![]() Complete the chart below. Use the chart above to help you determine the electron-pair and molecular geometries of the following molecules. When you choose the correct answer, the border around the answer will turn green. It will turn red if your answer is wrong.
Complete the chart below. Use the chart above to help you determine the electron-pair and molecular geometries of the following molecules. When you choose the correct answer, the border around the answer will turn green. It will turn red if your answer is wrong.
![]() Answer the following questions based on your answers to the activity above.
Answer the following questions based on your answers to the activity above.

![]() Using this interactive, compare the electron-pair geometries and molecular geometries of molecules in 3-D. You can manipulate the number of single, double, or triple bonds and lone pairs that are around a central atom. You can also see how the geometries are altered. Click on each model and move the molecule to see the various sides of the molecule.
Using this interactive, compare the electron-pair geometries and molecular geometries of molecules in 3-D. You can manipulate the number of single, double, or triple bonds and lone pairs that are around a central atom. You can also see how the geometries are altered. Click on each model and move the molecule to see the various sides of the molecule.